Objective:
Climbing chalk is often described as ‘high quality’, ‘best chalk’, ‘maximum grip’, ‘pure chalk’, ‘no impurities’, etc. As climbers, we’ve come to accept these qualitative statements but haven’t had the raw data to make our own decisions.
The goal of this project was to work with a university to study a wide selection of chalk options on the market and do standard industry mineral testing. We wanted to know what IS the composition of each of these chalks. Do high price chalks have a higher purity? How can we measure chalk friction in a lab environment? When considering chalk sourced from the ground (mined) versus synthesized from magnesium captured from seawater, are we making any concessions regarding purity or friction performance?
This project is intended as a data-gathering mission only. We are not looking to bias this data towards any opinions. Rather we are simply curious climbers, and we want to share this data and invite critical questions and discussion. As such, all brand names are blanked out and will remain anonymous. The goal here is simply to provide data for the climbing community to digest, question, and use the data to make decisions for themselves.
You’ll find two samples that were used as benchmarks and are not climbing chalks but represent raw materials. 22_JL_FL_2 is raw magnesium carbonate ore (MgCO3) and 22_JL_FL_3 is magnesium oxide (MgO). These were both mined at one of the purest commercial mine deposits of magnesium carbonate in the world, here in British Columbia, Canada.
Please come along with us on this journey, and feel free to reach out to us with questions and discussions!


Experiment Methods:
To understand what the chalk is made of and some of its characteristics, we looked at the following topics. We also need to note that test methods have limitations. These are important to keep in mind to be able to correctly put the data in context. Again, our aim here is to provide transparency and not bias data for any marketing or other reasons. Rather we want to give the data to climbers to make their own decisions, so context and data limitations are very important.
-
Mineral composition
- Common industry standard method here uses X-Ray Diffraction (Rietveld method). Essentially each mineral splits X-rays into specific directions based on its crystalline structure and shape. By shooting an X-Ray beam at chalk and collecting/analysing the resulting beams and angles we can determine what the chalk is made of.
- Limitations: this test method works only on materials that have a crystalline structure. It can’t pick up or identify elements that aren’t crystalline. Examples of non-crystalline materials include organic material or liquids like honey or pine rosin.
- XRD Spike Method (with internal standard)
- We also did select testing using this method. Here a known amount of a different substance is added to the test sample. In this way we can determine the absolute % contribution by each mineral. By knowing the absolute value of all crystalline minerals we can also know the amount of amorphous (non-crystalline) material within the chalk, although not its specific composition.
- Common industry standard method here uses X-Ray Diffraction (Rietveld method). Essentially each mineral splits X-rays into specific directions based on its crystalline structure and shape. By shooting an X-Ray beam at chalk and collecting/analysing the resulting beams and angles we can determine what the chalk is made of.
-
Alkalinity
- While not a common characteristic to measure we simply wanted to chase down this rabbit hole. A balanced (neutral) value would be 7. Above 7 shows basic and below 7 shows acidic.
- This could be used for future exploration around the questions of chalk-to-skin interactions, irritation, etc.
- We used de-ionized water to set the base point. Then by adding chalk and stirring for a period of time the pH was checked again with a probe.
-
Morphology (shape)
- This is basically the shape of the chalk particles. By taking samples of the chalk and ‘gluing’ them to a slide we could look closely at the chalk. We did this using both a microscope, which can be seen with the naked eye, and an SEM (scanning electron microscope). The SEM is capable of looking much closer (higher zoom power) and can also identify minerals.
- Some of the questions we had here include: what does chalk look like at the micrometer scale? Do we see any connection between shape of particle and chalk friction?
-
Bulk Chemistry
- It’s been said that heavy metals are part of low quality or low cost chalks. We know that heavy metals are found in many of our foods and daily use products, but how much and how much does it vary among chalk brands? Can a low price chalk also offer a high purity of magnesium and a low presence of heavy metals?
- This was tackled using routine rock testing methods, namely assay burn test and mass spectroscopy.
Results:
-
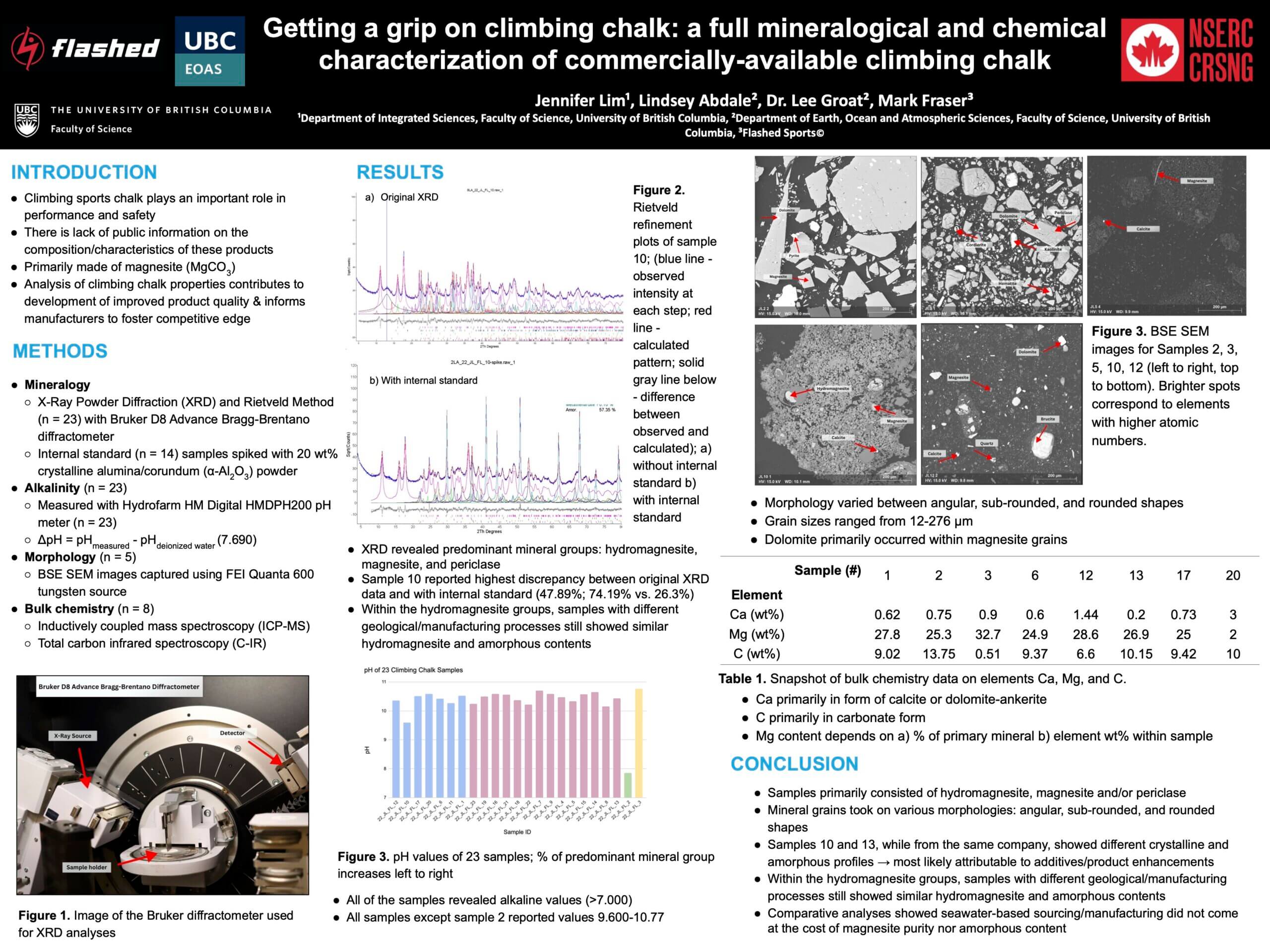
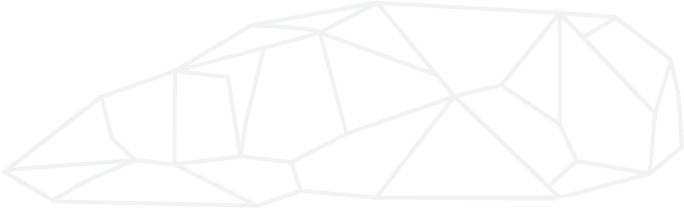
Mineral Composition using XR, Rietveld Method
- The first thing to note here is about the Rietveld Method. This method looks only at the crystalline structure material and not the amorphous (non-crystalline). This must be noted when looking at the % values of any mineral and kept in mind.
- Chalk sample results were broken up by color into 4 categories: blue (less than 90% hydromagnesite), purple (greater than 90% hydromagnesite), green (represents a benchmark material, 96% pure magnesite), yellow (represents another benchmark material, 98% periclase.
- Name Notes
- Magnesite
- This is the geological name for magnesium carbonate, MgC03.
- Hydromagnesite
- This is the geological name for hydrated magnesium carbonate, Mg5(CO3)4(OH)2-4H2O. This is colloquially what we know to be climbing chalk.
- Periclase
- This is magnesium oxide (magnesia), MgO.
- Magnesite
- Name Notes
- Through XRD we were able to name 22 different minerals across the whole set of tested chalks available on the market. While this may seem like a lot of different stuff it’s important to put that into context. We saw 61-99% of total crystalline content for the climbing chalk samples comprised of hydromagnesite with most above 88%.
-
Alkalinity
- Here we see very little variation among all climbing chalks. It should be noted that the only exception is sample 22_JL_FL_2 which is very close to neutral. Sample 22_JL_FL_2 also is not a climbing chalk, but rather a magnesium carbonate ore removed directly from the earth with no processing other than crushed to about a 1/16in diameter. In this form, it is not usable as a climbing chalk but makes a good benchmark for comparison.
- All results give a result of pH less than 11.
-
Morphology (shape)
- There was no preparation made for this. Chalk was simply scooped out of its packaging and put into a glue to be held in place for viewing and analysis. So this is a good way to see what the individual chalk particle shape and relative size is. As you can see some particles are both larger and more pointy (angular) than others. This may be important as it relates to friction.
- In the SEM photos you can also see little specs of base minerals that did not convert into hydromagnesite during the manufacturing process.
-
Bulk Chemistry (ingredients by periodic table of elements)
- When we burned select samples to identify elemental content, we see the main elements are magnesium and carbon. These two elements represent between 90-99% of total elements remaining after burning.
- Heavy metals (transition metals)
- Iron (Fe) – ranged between 0.01-0.19%
- Mercury – no samples showed any testable value (~0ppm)
- Since all other heavy metals amounts were very small they couldn’t be represented well-using %wt so instead we changed to ppm (parts per million).
- All tested brands varied from 91-134ppm (all heavy metals, except iron FE, summed together). Put differently this is varies from 0.0091-0.0135%
Stay tuned as we expand on these results!